Explore the essential ISO 14971 risk management process, a crucial framework for ensuring the safety and efficacy of medical devices. This guide provides a clear path to compliance and successful product development. Discover proven strategies to assess and mitigate risks effectively, safeguarding both patients and practitioners. From hazard identification to risk evaluation, gain invaluable insights that will enhance your grasp and implementation of the ISO 14971 risk management standards.
Elevate your medical device development process with this indispensable resource.
In this article
- What is the ISO 14971 Risk Management Model?
- Need for Risk Management in Medical Devices
- Example of an ISO 14971 Risk Management Model
- Basic Steps In The Medical Device Risk Management Process
- Create a Risk Management Model Easily Using EdrawMax
- Benefits of Using the ISO 14971 Risk Management Process
- Conclusion
Part 1: What is the ISO 14971 Risk Management Model?
The ISO 14971 Risk Management Model is a structured approach used in the development of medical devices. It helps identify and control potential risks to ensure the safety and effectiveness of the device. This model involves steps like assessing possible hazards, estimating the risks, and putting measures in place to minimize them. It's like a roadmap that guides manufacturers in making sure their medical devices meet high safety standards, providing confidence to both healthcare professionals and patients in their use.
Part 2: Need for Risk Management in Medical
Recognizing the critical importance of risk management in the field of medical devices is paramount. Here are key reasons why it plays an indispensable role in the development and deployment of these life-saving technologies:
- Patient Safety Assurance: Risk management ensures that medical devices are designed and manufactured with the highest regard for patient safety.
- Regulatory Compliance: It is a legal requirement in many countries to implement risk management processes for medical devices to gain regulatory approval.
- Minimize Product Liability: Effective risk management helps manufacturers identify and mitigate potential issues, reducing the likelihood of product recalls or lawsuits.
- Enhanced Reputation: Rigorous risk management practices build trust among healthcare professionals and patients, bolstering a company's reputation in the industry.
- Cost-Effective Development: Identifying and addressing risks early in the development process can lead to cost savings by preventing costly redesigns or recalls later on.
Part 3: Example of an ISO 14971 Risk Management Model
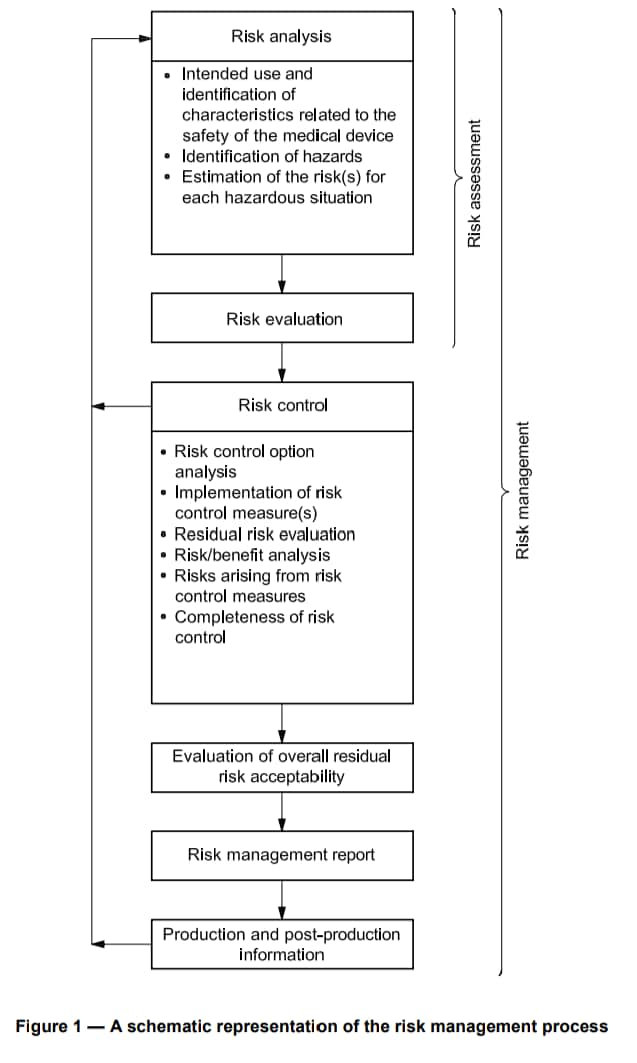
The ISO 14971 Risk Management Model is a rigorous framework tailored for the medical device industry. It involves a meticulous process of risk assessment, evaluation, and control at every stage of a device's life cycle. This encompasses comprehensive hazard identification, wherein potential risks are identified and categorized. Subsequently, each risk undergoes a detailed evaluation, considering factors like severity, probability, and detectability.
Following this, risk control measures are implemented, aiming to either eliminate or mitigate identified hazards.
Part 4: Basic Steps In The Medical Device Risk Management Process
Executing an effective risk management process is paramount in ensuring the safety and efficacy of medical devices. Here are the fundamental steps that constitute this critical process:
- Hazard Identification: Thoroughly analyze the device to identify potential hazards or failure modes.
- Risk Assessment: Evaluate each identified hazard based on factors like severity, probability, and detectability.
- Risk Control: Implement measures to either eliminate or mitigate identified risks.
- Residual Risk Evaluation: Assess the remaining risks after applying control measures.
- Risk Management Report: Document all findings, assessments, and control measures for future reference and regulatory compliance.
- Post-Market Surveillance: Continuously monitor the device's performance in real-world scenarios and update risk assessments as necessary.
Part 5: Create a Risk Management Model Easily Using EdrawMax
EdrawMax is the secret weapon for crafting a dynamic risk management framework! Its intuitive interface and ready-to-use templates make risk assessment a breeze. Drag-and-drop elements let you visualize complex scenarios effortlessly. Plus, it fosters seamless team collaboration, ensuring everyone's in sync on risk strategies. With multiple export options, it's a game-changer for accessibility. Elevate your risk management with EdrawMax – where innovation meets simplicity!
Here are the steps to create a risk management model using EdrawMax:
Step1
Open the EdrawMax software on your computer. Switch to the “Template” section and choose a suitable template for risk management. EdrawMax offers a variety of templates related to risk assessment and management.
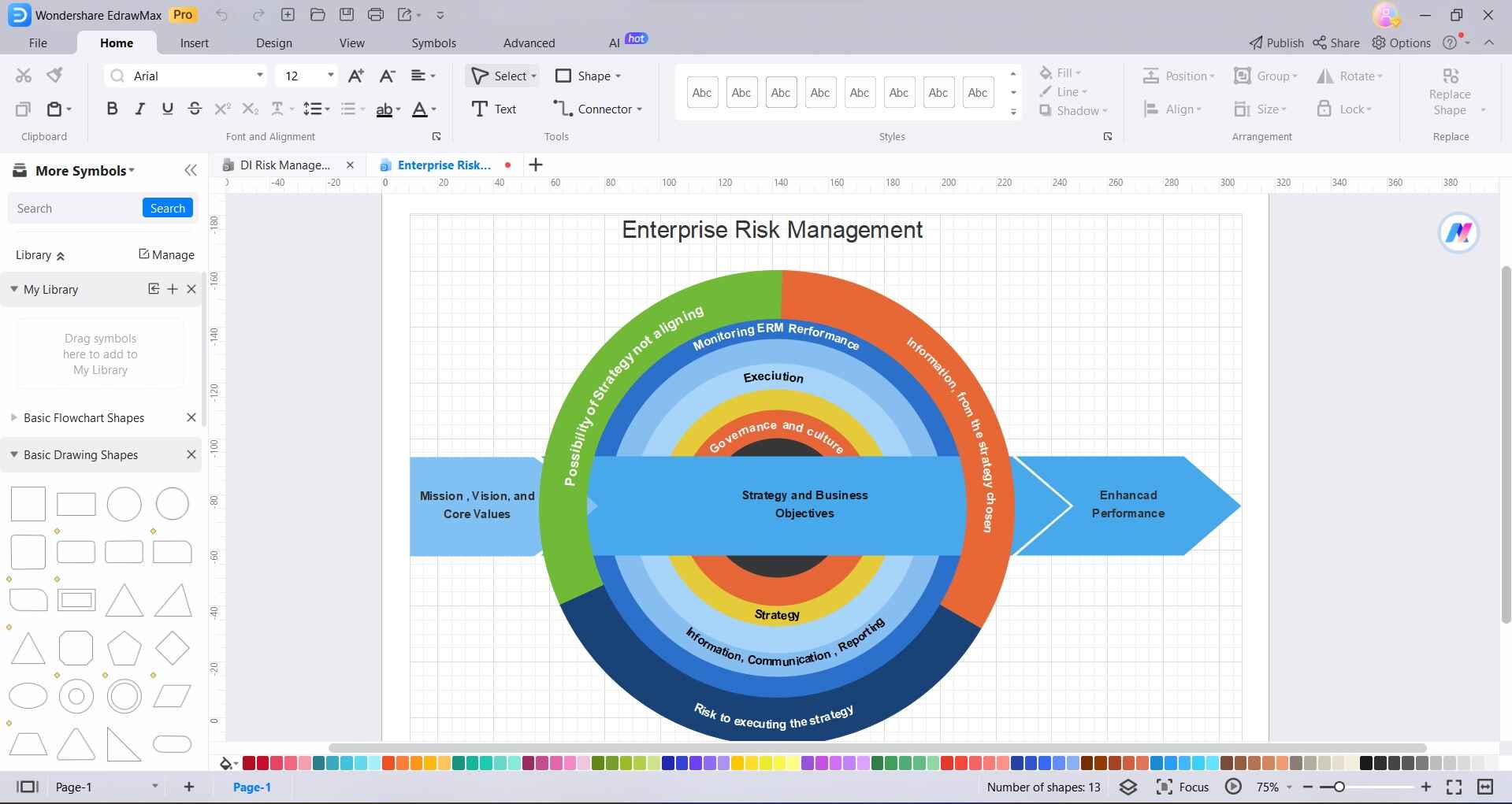
Step2
Drag and drop shapes, symbols, and elements onto the canvas to represent different aspects of your risk management model. These can include risk categories, assessment criteria, mitigation strategies, and more.
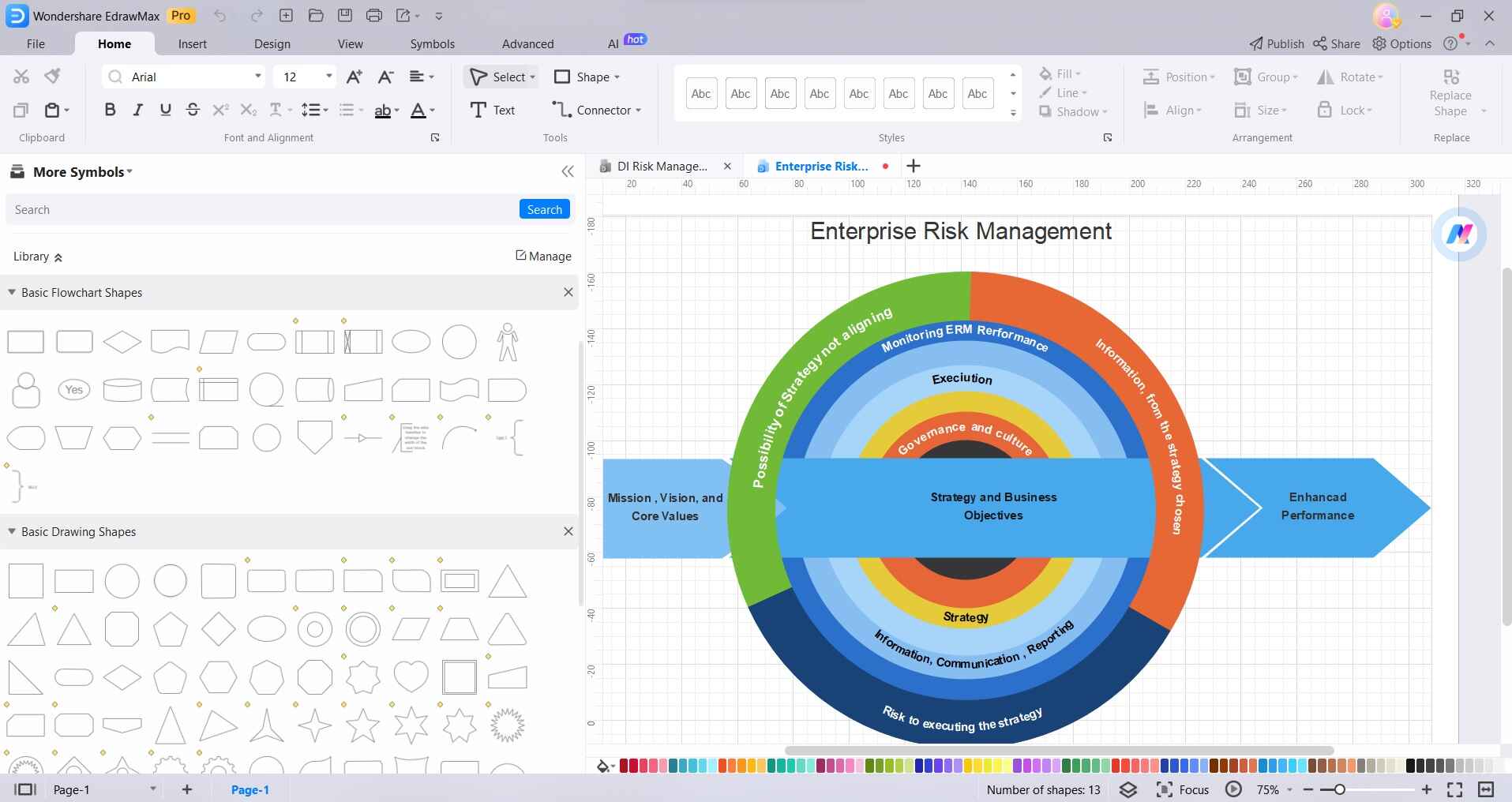
Step3
Use connectors or arrows to link different elements together, showing the flow of your risk management process. This helps in visualizing the relationships between different aspects of risk assessment and mitigation.
Step4
If applicable, include charts, graphs, or other visuals to illustrate data or statistics related to risks and their management.
Step5
Once you're satisfied with the model, save it to your preferred location on your computer for future reference.
Part 6: Benefits of Using the ISO 14971 Risk Management Process
Implementing the ISO 14971 Risk Management Process brings several crucial benefits. Here are some of the key advantages:
- Enhanced Patient Safety: Rigorous risk assessment and control measures lead to safer and more reliable medical devices.
- Regulatory Compliance: Adhering to ISO 14971 risk management standards ensures alignment with global regulatory requirements, facilitating smoother market access.
- Reduced Liability and Recalls: Proactive risk management minimizes the likelihood of product recalls and legal liabilities, saving both reputation and resources.
- Streamlined Development: By identifying and addressing risks early, the development process becomes more efficient, reducing time-to-market and associated costs.
- Improved Reputation: Implementing ISO 14971 demonstrates a commitment to quality and safety, enhancing trust among healthcare professionals and patients alike.
Conclusion
To sum up, understanding and implementing the ISO 14971 Risk Management Process is crucial for safe and effective medical devices. It guides manufacturers in identifying and managing risks throughout a device's life. This not only keeps patients safe but also helps meet legal requirements. Plus, it makes development more efficient and reduces potential problems.
Ultimately, it's a pledge to deliver top-notch, secure medical devices that instill trust.




